Mechanism of action
Melphalan flufenamide, also known as melflufen, is a first-in-class peptide-drug conjugate (PDC) in multiple myeloma that brings cytotoxicity into the tumour cells.
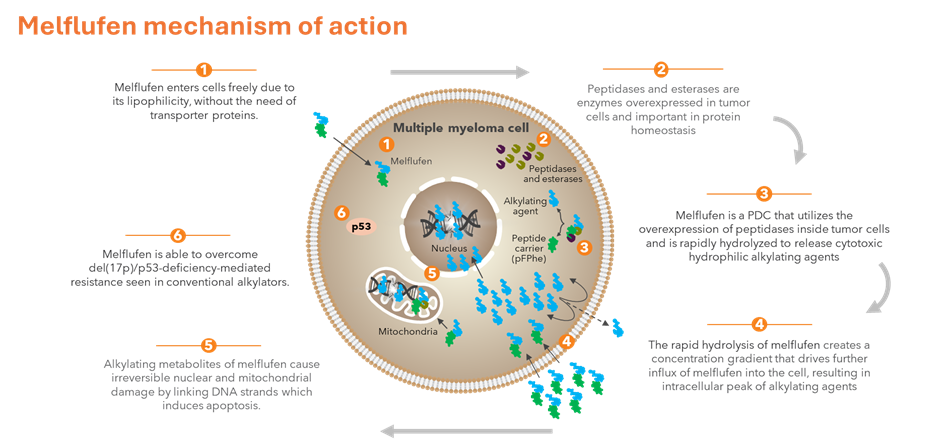
Melflufen enters cells freely due to its lipophilicity, without the need for transporter proteins. Melflufen is rapidly hydrolyzed by peptidases and esterases, which are overexpressed in tumor cells. The hydrolysis creates a concentration gradient that drives further influx of melflufen into the cell, resulting in intracellular accumulation of alkylating agents. This leads to rapid and irreversible nuclear and mitochondrial DNA damage and induction of apoptosis.1-5,9
- Melflufen is a first in class PDC in MM that, due to its lipophilicity, freely and rapidly crosses the cell membrane and therefore circumvents development of transporter-associated resistance.1-5
- Peptidases are enzymes that catalyze the hydrolysis of peptide bonds and are important in protein homeostasis.6,7 Aminopeptidase, a specific type of peptidase, is upregulated in cancer cells.2,7,8. In MM, the increased aminopeptidase expression is associated with advanced disease and a poorer outcome.9
- Esterases are also often overexpressed in cancer cells, linked to increased enzymatic activity in cancer cells.10
- Melflufen is efficiently and rapidly hydrolyzed by peptidases and esterases, resulting in a rapidly increased concentration of cytotoxic alkylating agents inside tumor cells.2,4,9
- The alkylating metabolites of melflufen (melphalan and desethyl-melflufen) induce irreversible DNA damage and apoptosis.1,5
- In vitro studies suggest that melflufen is 50-fold more potent than melphalan in myeloma cells due to the increase of intracellular alkylator concentration.2
- The cytotoxicity of melflufen is independent of p53 function.11,12
- Melflufen alkylates mitochondrial DNA thereby inducing mitochondrial dysfunction.12-14
- Melflufen suppresses myeloma clonal outgrowth in vitro.15
- Melflufen has demonstrated inhibition of metastatic processes and angiogenesis in vitro.16,17
Melflufen mechanism of action1,2,5,9,10,13,18
-
- Chauhan D, et al. Clin Cancer Res. 2013;19:3019-3031.
- Wickström M, et al. Oncotarget. 2017;8:66641-66655.
- Wickström M, et al. Biochem Pharmacol. 2010;79:1281-1290.
- Gullbo J, et al. J Drug Target. 2003;11:355-363.
- Ray A, et al. Br J Haematol. 2016;174:397-409.
- Taylor A. FASEB J. 1993;32:290-298.
- Holstein S.A., et al. Curr Cancer Drug Targets. 2023;23(1):25-46.
- Hitzerd SM, et al. Amino Acids. 2014;46(4):793-808.
- Miettinen JJ, et al. Cancers. 2021;13(7):1527.
- Kumari R, et al. Br J Cancer. 2021;124(8):1428-1436.
- Slipicevic A, et al. Poster presented at: American Association for Cancer Research (AACR) Annual Meeting 2020; June 22 – 24, 2020; Virtual Presentation. Poster 1843.
- Klára Ács et al. Presented at: 10th World Congress on Controversies in Multiple Myeloma; May 23-26, 2024; Paris, France & Online.
- Westermark U, et al. Biochem Biophys Res Commun. 2023:656:122-130.
- Haney et al. Poster presented at: the 26th Congress of the European Hematology Association (EHA) 2021; June 13-19, 2021; Virtual Presentation. Poster EP945
- Byrgazov K, et al. ASH 2020. Abs 2274
- Strese S, et al. Biochem Pharmacol. 2013; 86:888-895.
- Byrgazov K, et al. Poster presented at: European Society of Medical Oncology (ESMO) Annual Meeting; September 27-October 1, 2019; Barcelona, Spain.
- Mateos MV, et al. ASH 2020. Poster 3237.
